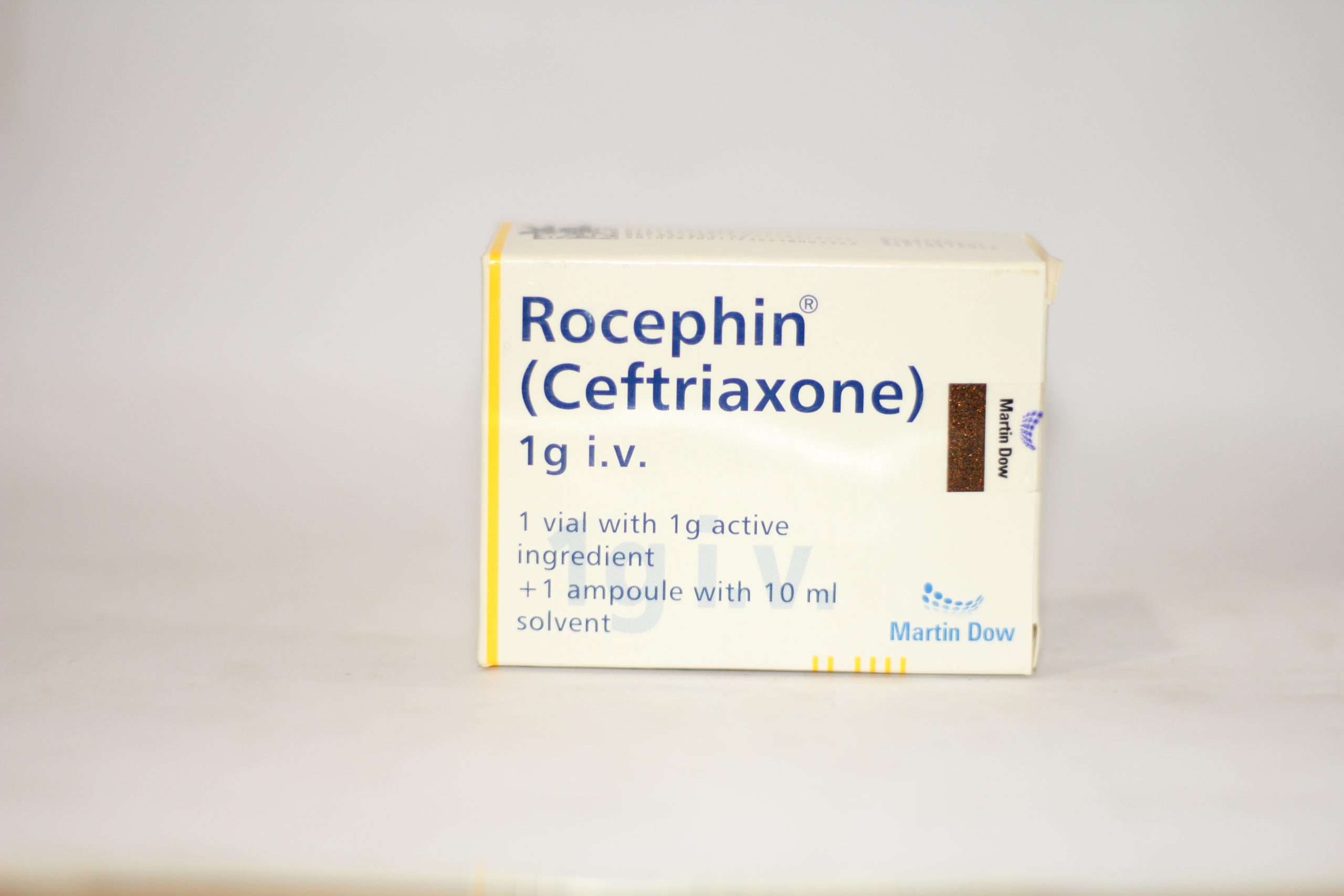
Why Was Rocephin Discontinued - Pfizer had ceftriaxone injection on shortage due. Fda intends to update this when additional information or analyses become available. Mar 15, 2025 · the food and drug administration (fda) has determined rocephin (ceftriaxone sodium) injection, 250 milligrams (mg), 500mg, 1 gram (g), 2g, and 10g base/vial, approved under new drug application (nda) 050585, were not withdrawn from sale for reasons of safety or. Ceftriaxone is used to treat the following bacterial infections: Ceftriaxone was not removed to any significant extent from the plasma by hemodialysis. In 6 of 26 dialysis patients, the elimination rate of ceftriaxone was markedly reduced, suggesting that. Rocephin Zur Infusion 2 g 1 St mit dem E-Rezept kaufen - Shop Apotheke
Pfizer had ceftriaxone injection on shortage due. Fda intends to update this when additional information or analyses become available. Mar 15, 2025 · the food and drug administration (fda) has determined rocephin (ceftriaxone sodium) injection, 250 milligrams (mg), 500mg, 1 gram (g), 2g, and 10g base/vial, approved under new drug application (nda) 050585, were not withdrawn from sale for reasons of safety or. Ceftriaxone is used to treat the following bacterial infections: Ceftriaxone was not removed to any significant extent from the plasma by hemodialysis. In 6 of 26 dialysis patients, the elimination rate of ceftriaxone was markedly reduced, suggesting that.
Ceftriaxone was not removed to any significant extent from the plasma by hemodialysis. In 6 of 26 dialysis patients, the elimination rate of ceftriaxone was markedly reduced, suggesting that. Mar 15, 2025 · the food and drug administration (fda) has determined rocephin (ceftriaxone sodium) injection, 250 milligrams (mg), 500mg, 1 gram (g), 2g, and 10g base/vial, approved under new drug application (nda) 050585, were not withdrawn from sale for reasons of safety or. On 23 january 2025, the european medicines agency completed a review of rocephin. The brand rocephin has been discontinued in the u. s. , however, generic ceftriaxone is available.

Mar 15, 2025 · the food and drug administration (fda) has determined rocephin (ceftriaxone sodium) injection, 250 milligrams (mg), 500mg, 1 gram (g), 2g, and 10g base/vial, approved under new drug application (nda) 050585, were not withdrawn from sale for reasons of safety or.
Best Restaurants Princeton Nj Myinprsretirement Org Login Ralph Fiennes